Lupine Publishers Group
Lupine Publishers
Menu
Review Article(ISSN: 2641-6875) 
The Influence of the Raw Materials used in the Ethanol Fermentation Over the Biogas Production from the Residual Vinasses Volume 3 - Issue 2
Yaniris Lorenzo1*, Lixis Rojas1, Fidel Domenech1, Oscar Almazán1, Juana Chanfón1, Magdalena Lorenzo1 and Felipe Eng1
- 1Research Institute of Sugarcane By-products, San Miguel del Padrón, Cuba
Received: October 20, 2022; Published: November 10, 2022
*Corresponding author: Yaniris Lorenzo, Research Institute of Sugarcane By-products, San Miguel del Padrón, Cuba
DOI: 10.32474/CTBM.2022.03.000157
Abstract
In the anaerobic treatment of the residual vinasses from the ethanol distilleries, the high sulfate content of them tends to lower the methane content of the biogas produced. This paper presents an initial assessment of the technical, economic and environmental impact of the use of non-sulfur-hued raw materials used in the ethanol production process on the sulfate concentration in the distillery vinasses, without any negative influence over the main parameters of the ethanol synthesis. The results of the experiments, carried on at a reduced scale (I L), give a reproducible indication of the best alternative was to use ammonium phosphate and urea as P2O5 sources and phosphoric acid to control the pH during the fermentation; this option gives a fermentation efficiency of 85.81%, an ethanol concentration of 32.27 g/100 ml, with a vinasses as residue with only 1893 mg of sulphate/ L. An scale-up of 60 folds (60 liters) of the selected Alternative 8 was carried on. A high replication of the results and the corresponding benefits of a large volume operation were obtained.
Keywords: Ethanol fermentation; distillery wastes; anaerobic digestion; biogas; methane content
Introduction
Ethanol fermentation process has as its main effluent the “distillery vinasses” a very aggressive residue. Recent studies confirm that the most viable way to treat vinasses is anaerobic bio digestion, to obtain biogas with a good energy potential [1,2]. However, the sulphates (SO42) content of the vinasses inhibits the efficiency of anaerobic digestion, inducing also a lowering of the methane content in the biogas and a significant presence of the very corrosive H2S, [3,4]. In this research work the influence of the use of non-sulfur nutrients in the ethanol biosynthesis over the composition of the vinasses waste, was studied, at a reduced reproducible level (1 L), allowing a very useful approach.
Experimental Procedures
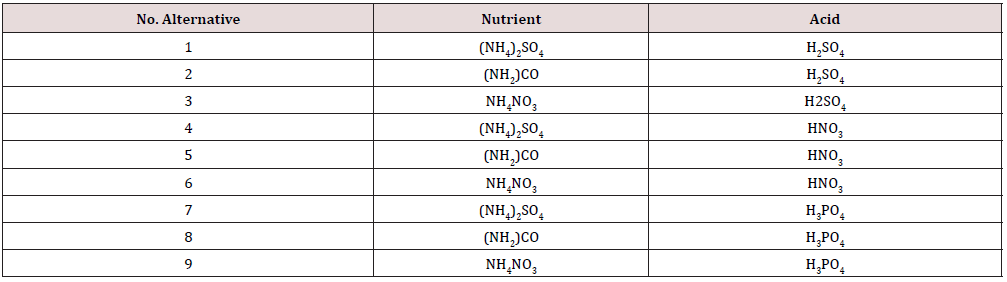
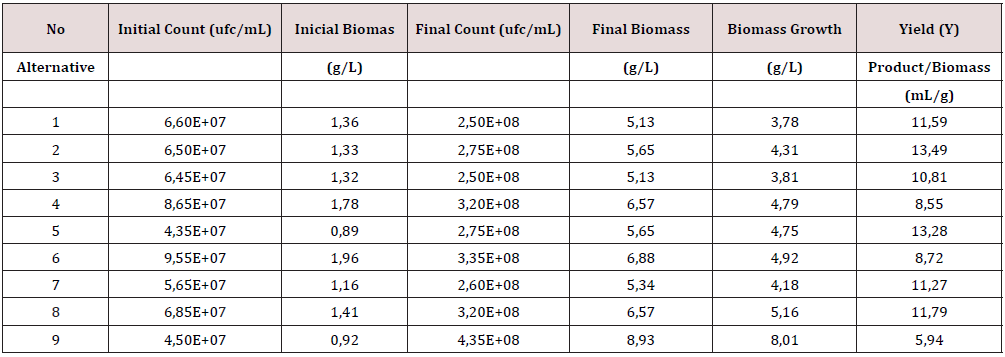
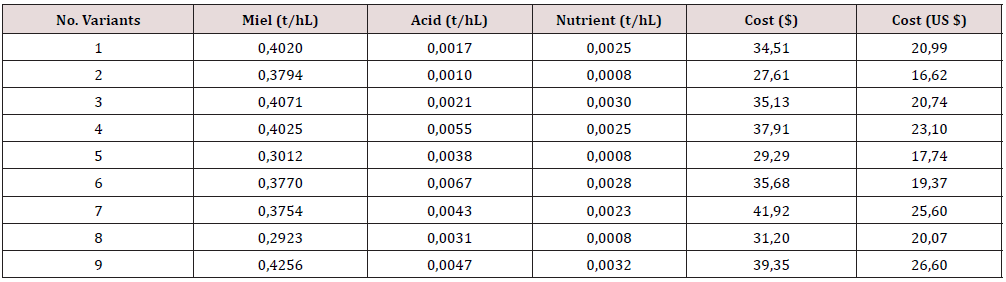
The study was carried using a 32 experimental design to evaluate the effects of the type of nutrients and acids used in the ethanol fermentation media (sulfur and non-sulfur compounds), at 1L experimental level, with B cane molasses with 66.63 g of total reducing sugar/g as the carbohydrates source, ammonium phosphate being the N and P2O5 nutrients and the strain L/25-7- 12 of Saccharomyces cerevisiae, commonly used in the ethanol commercial processes. To test the influence of the different nutrients salts, the fermentation medias were prepared using: 0.51 g/L of NH4HPO4; 1.39 g/L of (NH4) SO4; 0.63 g/L of (NH)2CO and 1.68 g/L of NH2NO3, to ensure the reported molecular composition of the Saccharomyces strain of CH1.64 N0.16 O0.52v P0.01 S0.005 [5]. The pH in all the medias tested were adjusted at 4.5 [6] with the acid selected for each test (Table 1); the medias were sterilized at 121°C for 45 minutes, cooled at 30°C, inoculated with a pure Saccharomyces strain in a relation v/v of 1/10, incubated for 48 hours without mechanical agitation. After 48 hours the fermented mash was distillated in a semi continuous set, shown in Figure 1, at 100 °C; samples of 150 ml of the condensate were collected and evaluated, the residual, as the equivalent vinasses, after de distillation were recovered from the distillation set.
In each experimental test run, the controlled parameters were:
a) For the inoculums: the cell count in a Neubauer chamber, using an optical microscope [7]; for the biomass growth the correlation of 4.87. 1010 cells /g of dry weight was used [8].
b) At the fermentation runs: Total Reducing Sugars (TRS) at the beginning and at the end of each cultivation test [9]; the ethanol content (°GL) of the fermentation mash, according to the [10] and the efficiency of the fermentation process, calculated by the relation between the ethanol produced and the true sugar consumed [11].
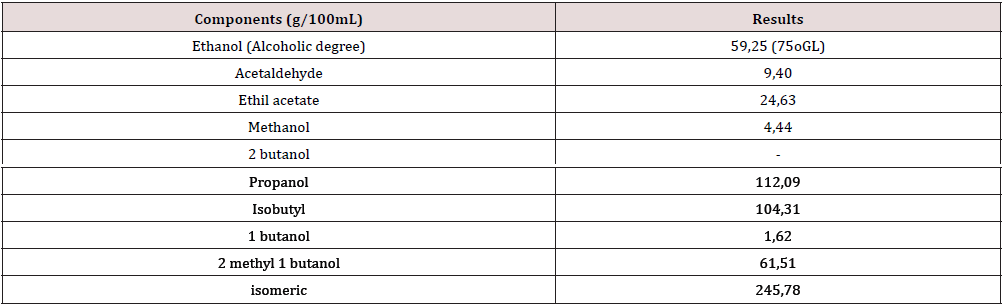
c) At the distillation: Ethanol and other compounds by capillary gaseous chromatography, by the extreme standard calculation (3-pentanol). The congeners were calculated taking as reference the alcohol content of the aguardiente (75%), for an equivalent of 59.25 g/100 ml as per the Cuban Standards [12].
d) The residual vinasses: were characterized following the standard methods for water and spends waters [13].
e) The statistical processing of the results: The results were processed by the multifunctional variance analysis (ANOVA), using the Stat graphic Centurion XV, 15.1.0.2, software [14].
f) The economic analysis: To select the most economically attractive alternative, a cost data was calculated for the ethanol recovered, based on 1 L, as an orientate behavior, taking the alternative 1 in Table 1 as reference, because its correspondence with the nutrients and acid most used in the local industry.
Results
In Ethanol Fermentation
For the pH adjustment of the fermentation the sulfuric acid was the one with the lower consumption, the nitric acid shows the highest with also the highest costs.
The Cell Count at the Ethanol Fermentation
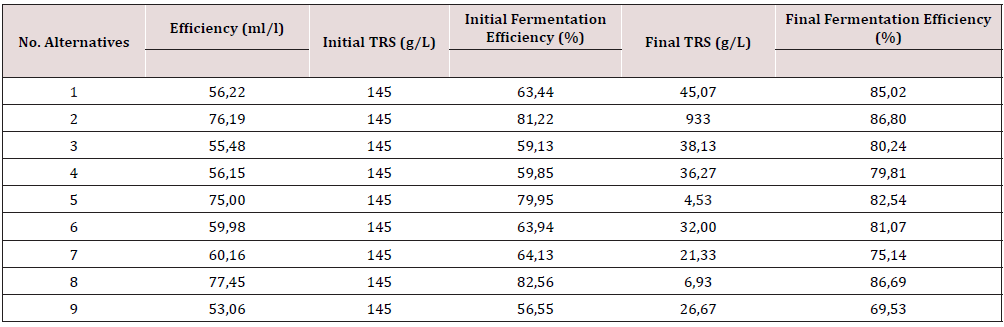
The results of the cell growth during the fermentation are shown in Table 2, the initial and final biomass was calculated by cell count. The biomass concentration at the end of the cultivation corresponds with the previous reported values reported in the literature (Table 3) [15]. The Yields product-biomass (ml ethanol/g) are in the range of the conventional values for ethanol fermentation processes, using cane molasses as a carbon source. The alternatives using urea as the N source (alternatives 2, 5 and 8) gave the highest yields.
The Fermentation Efficiency
The yields and the fermentation efficiency are given in Table 3. From Table it´s possible to appreciate that alternatives 2, 5 and 8 show the higher ethanol content per L of media and when urea was used as the N source; also, alternative 8 gave the higher ethanol production (77.45 ml/l), being the one where phosphoric acid was used for pH control. On the other hand, no significant differences in the ethanol bioconversion were appreciated in the alternatives tested; but alternatives 2 and 8, using urea, showed the best behavior. The statistical results confirm that the nutrients have a decisive impact over the fermentation efficiency and that only the urea has a beneficial influence over the ethanol bioconversion. In the figures in Tables 2 & 3 is possible to conclude that the best yield product – biomass is obtained with urea as the N source, as well as the best fermentation efficiency and the highest ethanol production, so being the best alternative.
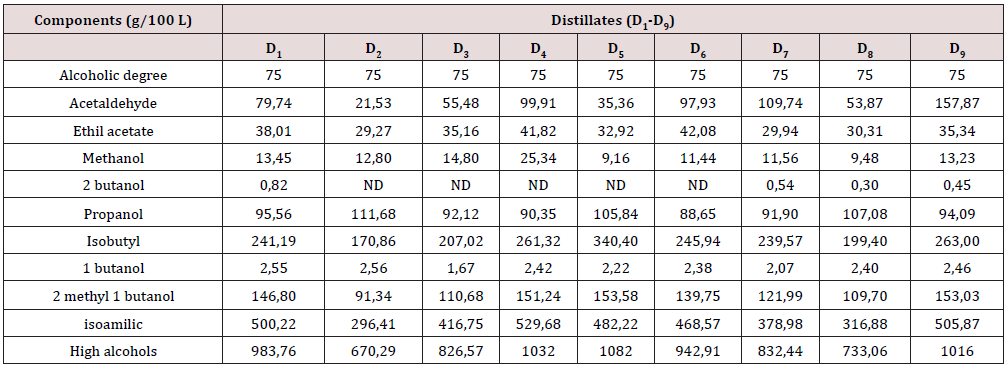
Characterization of Ethanol Distillates
In Table 4 it´s possible to have the full composition of all the samples of the distillates obtained during the tests. The esters contents, in all the samples, were under 50 g/100 L; the range of the high alcohols content (propanol, isobutyl alcohol, 2 methyl 1 butanol and isoamilic alcohol) was within the values of the Cuban Standard [12], (175 and 350), except for the isomeric alcohol. Note that the distillate samples D2 and D8, are under the referred values; with respect to the methanol content, it is evident that only samples D5 and D8, range below the established limit of 10 g/100; of all the distillates sampled in the tests, D8 has the most attractive composition to be used in beverages.
The Vinasses Composition
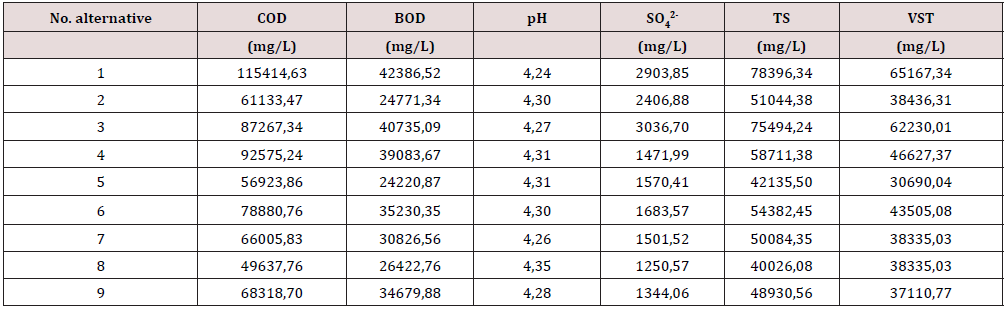
Table 5 shows the results of the influence of the raw materials over the vinasses composition [15,16]; different N and P2O5 sources were tested, also different acids for the pH control of the biosynthetic process, with the COD (chemistry oxygen demand), BOD (biological oxygen demand), pH, SO42w- (sulphate), as well as the Total Solids (TS) and Volatile Total Solids (STV) in the vinasses being the evaluated responds. It´s possible to appreciate that the relation TVS/TS was within the range of 0.75-0.96 in all the samples, a high COD value confirms their high organic matter content [17]; also, the pH values were between 4.20 and 4.35, typical values of the ethanol wastes. When Sulfuric acid is used to control the pH, during the ethanol biosynthesis, is possible to note a high sulfate content in the vinasses, the use of nitric acid for the pH control also induces a high sulfate content in the spends, the lowest values of sulfate in the vinasse are obtained when phosphoric acid regulates the pH of the fermentation. These results confirm the hypothesis that the sulfate content in the waste vinasses is highly dependable over the nutrients and the acid employed to control the pH of the biosynthesis. After a full evaluation of the alternatives tested, it´s clear that the best combination is the one using urea as a N source and phosphoric acid for the pH control.
Table 5. Characterization of the residual vinasses obtained in the test runs at the lab scale (1 L).
The Economic Analysis
Table 6 shows the total unit costs for all the alternatives studied taking in account [18]; the reports refer to the total cost and the foreign currency component of each one, per hl of ethanol. Alternative 1 was used as a reference, due to the fact that its nutrient pattern and acid used conventionally in the Cuban ethanol industry today. The analysis indicates that Alternative 2 has the lowest unit cost, but looking for an integral behavior, Alternative 8 combines a lower cost (of $ 31.20/hl and a component of US$ 20.07), a low sulfate content in the vinasses with a high ethanol yields.
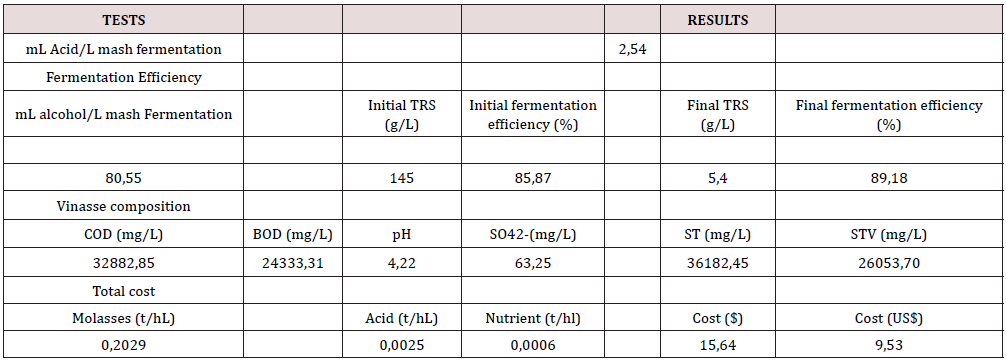
The Reproducibility Test
To confirm the results obtained and the confidence over the future scale-up of the evaluated hypothesis, and scale–up of 60 folds, of the selected Alternative 8, was carried out. The work done included the evaluation of the ethanol yields, the fermentation efficiency, the composition of the vinasses, the unit costs and the quality of the obtained ethanol distillates. The mean values are shown in Tables 7 & 8, referred to the main parameters evaluated.
Conclusion
The results indicate that when the selected waste vinasses treatment is anaerobic bio digestion, the best alternative is to use urea as the N source and phosphoric acid for the pH control of the ethanol biosynthesis. This combination guarantees a high ethanol fermentation efficiency, and the obtained distillates satisfy the Cuban Standard for the use in beverage. A scale-up of 60 folds of the selected Alternative 8 confirms a tight replication of the results and the corresponding benefits of working at large volumes.
References
- APHA, AWWA, WPCF (2000) Standard Methods for the examination of water and wastewater, 21 ed Washington DC.
- AZCUBA (2012) Input prices and cost sheet. Internal report.
- Bermúdez R (2000) Evaluation of the decrease in the contaminant load of distillery vinasse by anaerobic treatment. Rev. Int. Contam. Environment 16: 103-107.
- Cabrera A (2013) Vinasse treatment in an up flow packed anaerobic filter. Thesis in option to the degree of master’s in environmental engineering. ISPJAE Havana Cuba.
- Domenech F (1990) Continuous alcoholic fermentation with three reactors in series. Havana Cuban Institute for Research on Sugarcane Derivatives.
- Obaya M C (2004) Development of a technology for treating wastewater from alcohol distilleries. Final report.
- Frobisher M (1977) Culture and growth of bacteria. Microbiology second ed. SALVAT Barcelona.
- ICIDCA (1980) Manual of analytical techniques. second ed. Havana.
- NC: 290 (2007) Alcoholic beverages-determination of the alcoholic strength in spirits, distilled alcoholic beverages, wines, liqueurs, prepared alcoholic beverages, cocktails and hydroalcoholic extracts. Cuba.
- NC: 264 (2005) Aguardiente-Specifications. Cuba.
- Rojas L (2012) Influence of sulfur content in alcoholic fermentation and its impact on the concentration of sulfates in vinasses. Thesis in option to the master's degree in Engineering in biotechnological processes. ISPJAE, Havana, Cuba.
- Rojas L, Lorenzo Y, Doménech F (2011) Study of acid consumption in pH adjustment in different alcoholic fermentation media. ICIDCA About Deriv 45(2): 57-62.
- Russell I (2003) Understanding yeast Fundamentals. The Alcohol Textbook fourth ed. Nottingham University Press, UK.
- Shuler M (1992) Bioprocess Engineering Basic Concepts second ed. Prentice Hall NJ.
- Stat graphic Centurion XV, version 15.1.0.2 software. [Consultation: Jan.2013].
- Terreros J (2003) Two-stage anaerobic biodegradation of sodium alkylbenzene sulfonate in a UASB reactor. Thesis in option to the title of environmental engineer. DF Mexico Autonomous Metropolitan University 14(1): 57-64.
- Valdes A (2011) Residuals from alcohol production.
- Weiland P (2010) Biogas production: current state and prospects. Rev. Appl Microbiol Biotechnol 85(4): 849-860.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...